Abstract

Introduction: Treatment-free remission (TFR) in chronic myeloid leukemia (CML) is demonstrated to be achievable and recommended for patients (pts) in sustained deep molecular response (sDMR) who can discontinue tyrosine kinase inhibitor (TKI) treatment while maintaining responses. Studies investigating TKI de-escalation before TFR, mainly in CML chronic phase (CP) imatinib-treated pts, suggested that this stepwise approach is feasible. Despite nilotinib (NIL) is the first and only TKI approved for stopping therapy, nilotinib-based TFR optimization strategy has not been formally studied. Our phase II, prospective, multicenter DANTE study (NCT03874858) aims to shed light on frontline NIL de-escalation and successful TFR in Italian CML-CP patients. Our previously interim analysis showed that 1 year of NIL de-escalation before TFR in patients with sDMR is safe and effective. Here we present 1 year follow up results of those 40 patients who successfully completed NIL de-escalation and entered in TFR.
Methods: Adults patients with CML-CP treated with NIL 300 mg twice daily (bid) in first-line for ≥3 years who achieved sDMR for ≥1 year (≥MR 4.0; BCR-ABL level ≤0.01% IS) were enrolled in 27 centers. The study consisted of 4 phases: screening (week [wk] −4-0), consolidation (wk 0-48), TFR (wk 48-144), and follow-up (until wk 144). Ongoing treatment with ≥400 mg/day dose was allowed at study entry. During consolidation pts were treated with NIL 300 mg once daily (qd). At the end of consolidation phase, pts with sDMR entered TFR phase and NIL was discontinued. Patients with at least major molecular response (MMR; BCR-ABL ≤0.1% IS), but without sDMR, continued NIL 300 mg qd. At any time, pts with loss of MMR returned to NIL 300 mg bid. During TFR phase, BCR-ABL levels were monitored monthly from wk 52-96, and then every 3 months. Here we report updated results collected until the cut-off date of 8th February 2022, at which time all patients who entered the TFR phase had completed at least one year of TFR, or switched to full-dose phase, or entered in survival follow-up or discontinued the study.
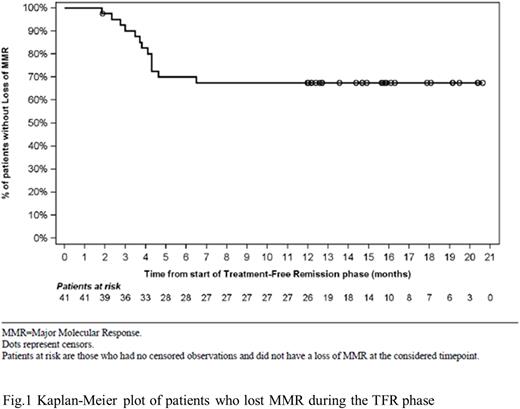
Results: Overall, 113 pts were screened and 107 entered in consolidation phase. The baseline characteristics and the clinical outcome of the 52 patients that reached the end of consolidation phase by the first data cut-off period (February 8, 2021) have been previously reported (Breccia et al., 2021). Specifically, 47 out of 52 pts completed the consolidation phase and 40 (76.9%) of them, with sDMR, entered in the TFR phase. At 1-year data cut off, with a median duration of 12.7 months (range 1.9 - 20.6), 27 out of 40 pts (67.5 %) who entered in the TFR phase remained in MMR or better while 13 pts (32.5%) had discontinued the TFR phase (11 pts due to loss of MMR and 2 pts for subject/guardian decision). The median time spent in TFR phase until loss of MMR was 3.6 months (range 2-7 months). The median time to loss of MMR in TFR phase was not reached (Fig 1). At the data cut off, all patients except one (10/11, 90%) who lost MMR, regained MMR after NIL resumption. Overall, among the 13 patients who restarted NIL after MMR loss (2 pts from consolidation phase and 11 from TFR phase), 12 of them (92.3%) regained MMR and MR4 after a median time of 2.6 months (range 0.9 - 5.2) and 4.2 months (range 2.7- 8.0), respectively. Notably, 22/52 (42%) pts remained in DMR from consolidation phase until week 96.
During the TFR phase, all grade AEs were reported in 26/40 (65%) pts. One pt (2.5%) had serious AE (pneumonia). No deaths and disease progressions were observed.
Conclusions: Our finding show that approximately 68% of pts remained disease-free at 1 year after stopping NIL, indicating that de-escalation of NIL before TFR attempt in CML-CP pts with sDMR can be a successful dose optimization strategy.
Reference: Breccia et al., Blood (2021) 138 (Supplement 1): 1474. https://doi.org/10.1182/blood-2021-145411
Disclosures
Stagno:Celgene, Incyte, Novartis, Pfizer: Honoraria, Speakers Bureau. Abruzzese:BMS, Incyte, Novartis, Pfizer: Consultancy. Iurlo:Novartis, BMS, Celgene, Incyte, Pfizer: Honoraria. Pane:Novartis: Research Funding, Speakers Bureau. Galimberti:Abbvie Incyte, Novartis, Janssen, Astrazeneca, Pfizer: Honoraria. Scappini:Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees. Siragusa:Csl Behring, Takeda, Amgen, Novartis, Bayer, Sobi, Novo Nordisk: Honoraria. Capodanno:Novartis, Incyte, Pfizer, Celgene, IMS Health: Consultancy, Honoraria. Valsecchi:Novartis: Current Employment. Nardozza:Novartis: Current Employment. Coco:Novartis: Current Employment. Rosti:Novartis, Incyte, BMS, Pfizer: Honoraria, Speakers Bureau. Saglio:Novartis: Speakers Bureau. Breccia:Novartis, Incyte, Pfizer, BMS, Abbvie: Honoraria.
OffLabel Disclosure:
Reduced dosage of nilotinib (Tasigna) compared to the approved one
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal